FAQ
Q: Rust has appeared.
A:
Stainless steel is not a completely rust-free metal. However, it is a rust-resistant metal. The reason stainless steel is rust-resistant is because it is covered by a thin film called a “passive film.”
This film is formed when the chromium contained in stainless steel reacts with oxygen in the air.
This film is naturally generated as long as the stainless steel is exposed to air, but rust can occur if the surface is covered (e.g., with dirt or residue) or damaged (e.g., by salt).
Please take care during cleaning and be sure to wipe off moisture before storing.
Q: There is a white deposit on the inside.
A:
This is caused by minerals (such as magnesium and calcium) contained in water adhering to the surface.
These minerals are naturally present in water and are not harmful to the human body.
Even tap water can leave deposits, but mineral water contains even more minerals, making it more prone to this issue.
To remove the deposit:
- Boil a diluted solution of citric acid (available at pharmacies, 500g for around ¥1,000).
(Fill the pot or kettle about halfway with water and add one capful of citric acid.) - Use a pot cleaning agent.
- Boil water with about 10% vinegar content.
- Boil a diluted solution of citric acid (available at pharmacies, 500g for around ¥1,000).
 Before boiling with citric acid
Before boiling with citric acid |
 After boiling with citric acid
After boiling with citric acid |
- 16cm pot: 5 tablespoons
- 20cm pot: 10 tablespoons
- 2.5L kettle: 8 tablespoons
Q: White spots have appeared.
A:
This is a type of rust known as “pitting corrosion.”
This phenomenon is especially caused by salt residue.
After cooking with salty ingredients, please wash thoroughly and wipe off moisture before storing.
Also, as chlorine is added to tap water for sanitation and disinfection, be sure to wipe off moisture after washing.
Although these white spots cannot be removed, continued use is not a problem.
 Example image of white pitting corrosion Example image of white pitting corrosion |
 Microscopic view Microscopic view |
Q: Brown spots have appeared.
A:
This is also a type of rust known as “pitting corrosion.”
When these brown spots progress, they turn into white spots (see previous item).
Please be extra careful during cleaning.
 Example image of brown pitting corrosion Example image of brown pitting corrosion |
 Microscopic view Microscopic view |
Q: The inside has discolored with a rainbow-like color.
A:
This phenomenon occurs when trace amounts of iron or copper contained in water are left behind as the water evaporates and adhere to the surface of the stainless steel, creating a rainbow-like appearance.
These are components naturally found in water and are not harmful to the body.
- Boil a diluted solution of citric acid (available at pharmacies, 500g for around ¥1,000). (Fill the pot or kettle about halfway with water and add one capful of citric acid.)
- Use a pot cleaning agent.
 Before boiling with citric acid Before boiling with citric acid |
 After boiling with citric acid After boiling with citric acid |
Estimated amount:
Fill the pot or kettle about halfway with water. Use the following amount of vinegar:
- 16cm pot: 5 tablespoons
- 20cm pot: 10 tablespoons
- 2.5L kettle: 8 tablespoons
Any of the above methods will remove the discoloration. Afterward, wash with dishwashing detergent and dry thoroughly.
Q: The glass lid broke.
A:
The causes of glass breakage include:
- Impact from dropping, etc.
- Sudden temperature change, such as immersing it in water while still hot
- It is possible that the glass was exposed to heat exceeding its heat resistance temperature.
Particularly in the third case, please be careful.
If the glass lid is placed off-center on the pot while heating, the metal rim may become overheated and crack.
 |
Q: Could the glass lid have broken due to heat?
A:
One possible cause is that the lid was exposed to heat exceeding its heat resistance temperature.
In particular, if the lid was positioned off-center and partially over a flame, the metal rim may become overheated and break. Please take care to avoid this.
Q: The fluororesin coating has peeled off.
A:
- Being heated to a temperature high enough to cause browning of the coating
- Scraping with a metal spatula or sharp object
- Exposure to heat exceeding the heat resistance temperature of the glass
 |
Q: Can I use pots or kettles in a dishwasher or dryer?
A:
Do not use products with plastic handles (grips or knobs) in dishwashers or dryers.
Degreasing and drying can cause the plastic to deteriorate.
 |
Q: I smell something like burnt plastic.
A:
Please check whether the handle is being scorched.
If so, it is likely due to deterioration of the resin.
Use heat that does not extend beyond the bottom of the pot.
 Flames touching metal can cause brown discoloration.
Flames touching metal can cause brown discoloration. |
Q: The handle is wobbly.
A:
Please check whether the handle is being scorched.
If the plastic is swelling or if the metal part is discolored brown, it is a sign that the heat is too strong.
Use heat that does not extend beyond the bottom of the pot.
If the handle is loose even though it is not scorched, tighten the screw if it is a screw-type handle.
If it is not a screw-type, please stop using the product.
 Continued use may cause breakage as deterioration progresses. Continued use may cause breakage as deterioration progresses. |
Q: Why does it say “Do not store food in the pot”?
A:
For general household pots, under the “Household Goods Quality Labeling Law,” there is a regulation called “Labeling Requirements for Miscellaneous Industrial Products.”
This regulation requires manufacturers to indicate that food should not be stored in the pot.
Although some customers may store food in the pot after cooking, we also advise against this because it can cause rust, depending on the type of dish or salt content.
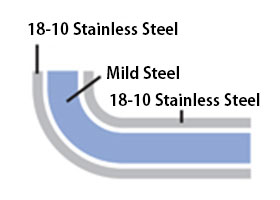
Q: The rim of my three-layer steel pot has rusted.
A:
Our three-layer steel products use iron sandwiched between stainless steel.
The edge of the material would rust easily if left exposed, so the rim is curled inward to prevent the iron from being exposed.
 | 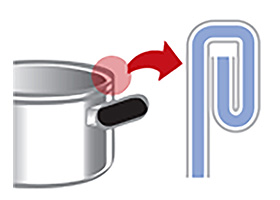 |
- The pot is left soaking in water
- The pot is stored upside down after washing
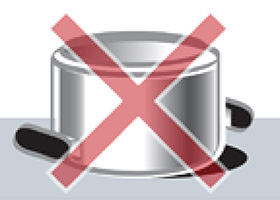 |
 Rust at the rim of a three-layer
steel pot Rust at the rim of a three-layer
steel pot |
Q: I can’t open the lid of the pot.
A:
This happens when the pot cools down with the lid on, lowering the internal air pressure and causing the lid to be pressed down tightly.
To open it, gently heat the pot over low heat. This will raise the internal pressure and allow the lid to come off.
If soup or broth has adhered between the lid and the pot, pour a little water on the affected area and heat it on low until the lid opens.
Q: The bottom of the pot deformed after stir-frying on an IH stove.
A:
Stir-frying can cause the bottom of the pot to deform, whether using IH or gas heat.
Why does this happen?
Normally, water only boils up to 100°C, even with ingredients inside. This prevents the pot body from overheating.
However, if there is no water in the pot (such as during dry heating or stir-frying), nothing prevents the temperature from rising, and the pot body will continue to heat up excessively.
As a result, the bottom of the pot may deform.
Since IH stove tops are flat, a deformed bottom can cause instability, make the pot harder to use, reduce cooking efficiency, and in some cases, render the pot unusable.
To ensure the pot lasts a long time, please refrain from using it for stir-frying.
 |
 Bulging caused by stir-frying Bulging caused by stir-frying |
Q: Food sticks to my iron frying pan.
A:
The most common reason food sticks is that the pan is not properly seasoned with oil.
To season the pan properly:
(1) Perform initial “burn-in” treatment before first use.
This process helps oil adhere to the pan.
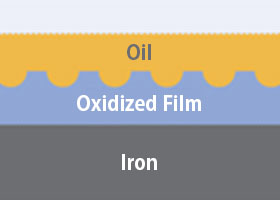
When iron is heated, a thin oxidized layer (oxide film) forms on the surface.
This film is porous, allowing oil to seep in and bind. Oil does not bond with the metal directly, but with this oxidized film.
Therefore, creating this film is essential. Please ensure the burn-in is thorough.
(2) Perform “oil return” before each use.
- Preheat the pan until it just begins to smoke.
(If it smokes too much, it’s overheated—turn off the heat temporarily.) - Add slightly more oil than you would use for stir-frying and let it coat well.
- Remove excess oil into an oil pot, then add the necessary amount of oil for cooking.
(3) Do not use dish soap after use.
Using detergent will wash away the seasoned oil.
Instead, soak in hot water, scrub with a sponge or “Kamenoko Tawashi,” then dry thoroughly and apply a thin layer of oil before storing.
Please pay attention to the following points.
The surface of a well-seasoned frying pan will feel smooth to the touch, even without oil applied.
If the oil is well absorbed to this degree, the frying pan becomes resistant to food sticking.
 |  |  |
Q: The pot turned brown after just one stir-fry.
A:
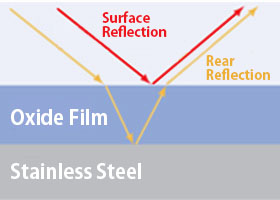
This discoloration is caused by light interference.
When the stainless steel surface is overheated, an oxide film forms due to the oxidation of iron.
The brown color appears because light reflects off both the surface of the film and its interior, causing interference.
 |
This color is known as a “temper color,” and the hue changes depending on the temperature:
| Color | Temperature |
| Pale yellow | 290°C |
| Brown | 340°C |
| Purple | 390°C |
| Violet | 450°C |
| Deep blue | 530°C |
| Light blue | 600°C |
This phenomenon will not occur when the pot contains water, since water boils at 100°C, keeping the temperature controlled.
However, when the pot is used dry (for stir-frying or empty heating), the temperature continues to rise without regulation, resulting in discoloration.
 A pot turned brown after one stir-fry A pot turned brown after one stir-fry |
 Pot repeatedly overheated when dry (partially discolored to light blue) Pot repeatedly overheated when dry (partially discolored to light blue) |
Also, if a pot is half-filled with water and the heat exceeds the base diameter of the pot, only the upper area that is not submerged will discolor, while the submerged part will not. (This applies to kettles as well.)
This discoloration is not harmful to the human body and does not affect usage.
If you wish to remove it, use stainless steel-specific polish available at hardware stores.
 Upper half of pot discolored Upper half of pot discolored |
 Discoloration removed with stainless steel polish Discoloration removed with stainless steel polish |
- Avoid dry heating and stir-frying
- Do not use heat that exceeds the bottom of the pot
About Light Interference
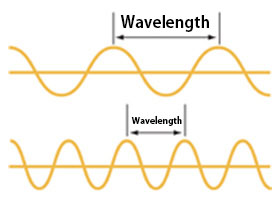
Light has wave properties.
The intensity of light is determined by the amplitude of the wave, and the color of light is determined by its wavelength.
When the wavelengths align, the light becomes stronger; when they do not, the light becomes weaker.
For example, “a red apple appears red” because only red light is reflected while other colors are absorbed.
The human eye recognizes color through combinations of the three primary colors of light: red, green, and blue.
When all three are combined at 100%, the result is white.
Wavelengths from longest to shortest:
Red → Orange → Yellow → Green → Blue → Indigo → Violet
Visible light has wavelengths in the range of 380–780 nm (nanometers; 1 nanometer = 1 millionth of a millimeter).
Light with a longer wavelength than red is called infrared, and shorter than violet is called ultraviolet.
Both are invisible to the naked eye.
 |
Q: What is a cooking heater?
A:
- Radiant heaters
- Halogen heaters
- Sheathed heaters
- Enclosure heaters
